Fortnightly PCa News
New tests aid decision making, New and Old Databases, things you can do to improve your odds.MiPS improves PSA sensitivity while reducing false alarms for biopsys
| Building a Better Mousetrap to catch Prostate Cancer - Prostate Cancer Foundation (PCF) |
A new urine-based test improved prostate cancer detection - including detecting more aggressive forms of prostate cancer - compared to traditional models based on prostate serum antigen, or PSA, levels, a new study finds.
The test, developed at the University of Michigan Comprehensive Cancer Center, is called Mi-Prostate Score, or MiPS. It combines PSA with two markers for prostate cancer, T2:ERG and PCA3, both of which can be detected through a urine sample. The test has been available clinically since September 2013.
After Surgery - New optical method for recurrence screening
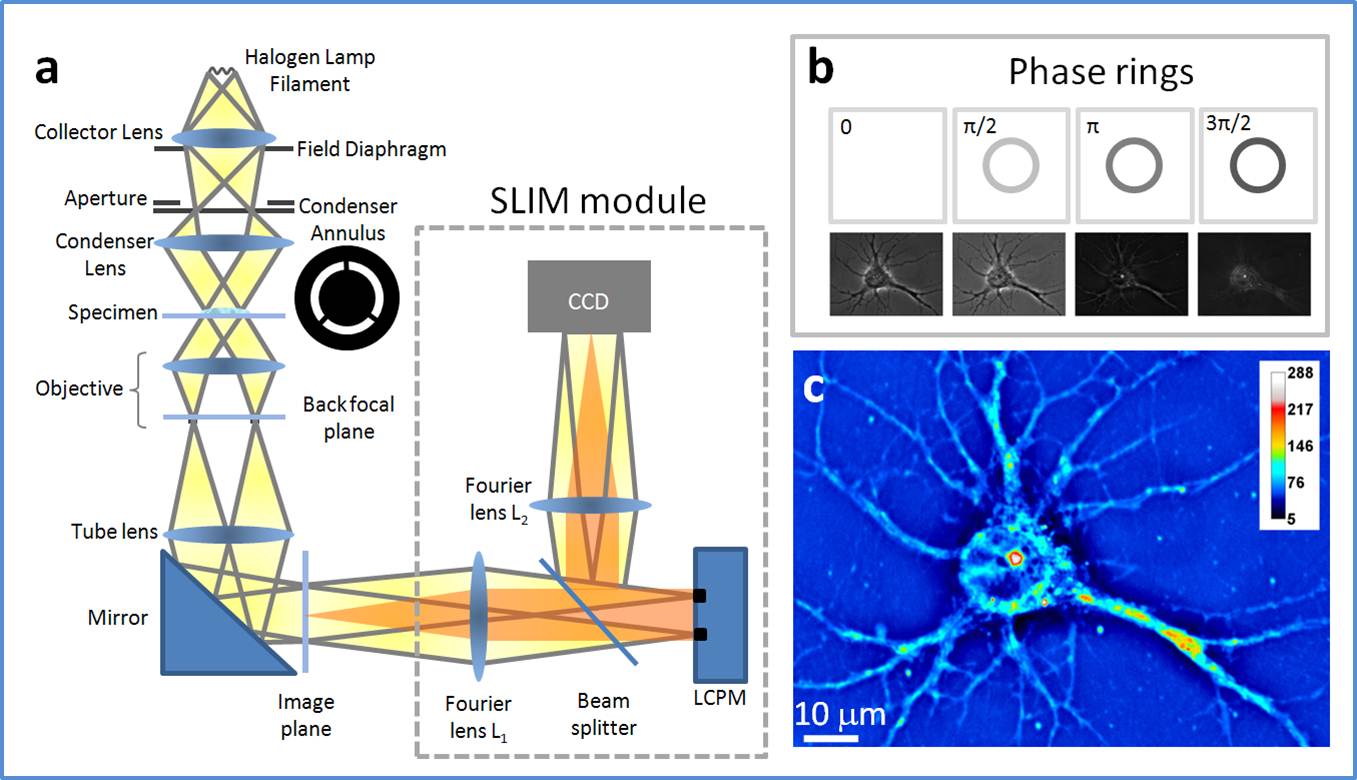 |
| Spatial Light Interference Microscopy (SLIM) | QLI |
On a study funded by the National Science Foundation and Agilent Technologies, researchers employed spatial light interference microscopy (SLIM), a label-free method, to perform localized measurements of light scattering in prostatectomy tissue microarrays. The quantitative phase imaging (QPI) performed by the SLIM examines the anisotropy, or the difference in a material's physical properties, as light is scattered through the stroma, the tissue surrounding the prostate glands. The results can be found in an article "Prediction of Prostate Cancer Recurrence using Quantitative Phase Imaging," published in Scientific Reports.
So you had a Biopsy with Gleason 7 - Genes may help decide to Cut or Zap?
 |
| The landscape of Gleason score 7 prostate cancer |
Researchers unveil new gene subgroup in prostate cancer -- ScienceDaily
An introduction to CPC-GENE - Prostate Cancer Canada
Spatial genomic heterogeneity within localized, multifocal prostate cancer : Nature Genetics : Nature Publishing Group
The discoveries, published online in Nature Genetics, are a further step along the road to personalizing prostate cancer medicine say study co-leads, Dr. Robert Bristow, a clinician-scientist at Princess Margaret Cancer Centre, and Dr. Paul Boutros, an investigator at the Ontario Institute for Cancer Research.
"Our research shows how prostate cancers can vary from one man to another - despite the same pathology under the microscope - as well as how it can vary within one man who may have multiple tumour types in his prostate," says Dr. Bristow. He goes on to say, "these sub-types may be important to determining the response to surgery or radiotherapy between patients."
Dr. Boutros, explained that the more detailed analysis clearly identified that two members of the MYC cancer gene family were at play in disease development, and that one of them - "C-MYC" - was the culprit driving aggressive disease. The other one - "L-MYC" - is already known to be implicated in lung and other cancers. "This discovery of a new prostate cancer-causing gene gives researchers a new avenue to explore the biology of the disease and improve treatment," says Dr. Paul Boutros, a principal investigator at the Ontario Institute for Cancer Research.
"By showing that mutations in prostate cancer vary spatially in different regions of a tumour, this study will aid in the development of new diagnostic tests that will improve treatment by allowing it to be further personalized."
Dr. Bristow says about half of all prostate cancer patients have either C-MYC or L-MYC mutations, but never both: "Our findings suggest we are getting closer to subtyping prostate cancer based on which gene is present to determine a patients' disease aggression in terms of the risk of spread outside the prostate gland at time of treatment. In developing this research tool into a clinical test within three years, we hope to inform doctors and patients about specialized treatments for each prostate cancer patient."
NCI quietly drops PSA test data from SEER database - files were inaccurate
Fallout from elimination of PSA data from the SEER database | THE "NEW" PROSTATE CANCER INFOLINKProstate-Specific Antigen (PSA) Testing - SEER Landmark Studies
On April 29 this year, the National Cancer Institute (NCI) announced a decision to eliminate all PSA data from their current data files because of apparent inaccuracies in the ways that such data had been accumulated over time and administered. The full ramifications of this are — as yet — unclear.In 2003, celebrating 30th anniversary - "Today, SEER stands as the model and standard of excellence for cancer registries, both on a national and international scale. Despite the enormous challenges involved in monitoring cancer in the large, mobile, and diverse U.S. population, SEER has succeeded in building an extraordinary resource that has in so many ways galvanized epidemiologic research into the causes and control of cancer. Visionary in concept, SEER has earned its name with an unprecedented ability to identify emerging trends, geographic variation, ethnic disparities, and other patterns that have provided new directions for epidemiologic research in cancer etiology and control."
- Joseph F. Fraumeni, Jr., M.D., Director, Division of Cancer Epidemiology and Genetics, NCI
If you want to read exactly why the NCI made this decision, click here. (There was no media release about this, so most of us didn’t hear about this until the last couple of days.)
If you want to be able to read what a well-respected specialist in urology thinks about it, click here The Power and the Peril of Large Administrative Databases.
Noted urologist calls attention to implications of flawed prostate specific antigen data in SEER - Medical News Today
If you want to read what Medscape had to say on the topic (complete with quotes from Dr. Penson’s article and the opinions of at least one other well-respected urologic oncologist), then click here Flawed PSA Data in SEER Make Past Studies Suspect. e.g.; ADT and radiation for first-line treatment of node-positive (N1) prostate cancer
While AUA launches AQUA PCa Registry
US national prostate cancer registry coming to life soonDr. Matt Cooperberg reported that a total of 450 US-based urologists had now signed up to contribute data on their prostate cancer patients to the AQUA Registry.
This is really good news for patients because it will help to accelerate adoption of more consistent and higher standards of care for all prostate cancer patients in America over time.
AUA Quality Registry (AQUA): American Urological Association
AUA’s national quality registry begins extracting data, continues to add sites | AUA Daily News
When developing the registry, the AUA built on experience gained from other prostrate cancer registries. What sets AQUA apart from previous efforts is an attempt to bypass a major obstacle of past registries — the need for manual data abstraction and entry. Instead, the AUA is using automated data extraction from electronic health records.
“Every previous registry has relied on somebody — a physician, a nurse, a paid abstracter — to go into the charts and pull out data,” Dr. Cooperberg said. “It’s slow, it’s cumbersome [subject to error] and the bottom line is that it’s prohibitively expensive for the AUA or anyone to really do that consistently and well on a national level.”
Green Tea Catechin extract helps lower PSA level
Component in green tea may help reduce prostate cancer in men at high risk, Moffitt Cancer Center researchers say - Medical News TodayThe goal of this trial was to evaluate if a one-year intervention with green tea catechins could suppress prostate cancer development in men who had high-grade intraepithelial neoplasia (HGPIN) or atypical small acinar proliferation (ASAP). The researchers used decaffeinated green tea capsules called Polyphenon E that contained a mixture of catechins that predominantly contained EGCG at a dose of 200 mgs twice a day.
The researchers compared Polyphenon E in 49 men to placebo tablets in 48 men over a 1 year treatment period. Overall, the difference in the number of prostate cancer cases at the end of 1 year between the two treatment groups was not statistically significant. However, in men who only had HGPIN at the beginning of the trial, they observed a lower combined rate of ASAP and prostate cancer development with Polyophenon E. ASAP is an entity that reflects a broad group of lesions in the prostate with insufficient changes in the cells to be definitively diagnosed as prostate cancer. Additionally, men on Polyphenon E had a significant decrease in prostate-specific antigen (PSA) levels. PSA is a biomarker that in combination with other risk factors is used to screen patients for prostate cancer, and high levels signify a higher risk of prostate cancer.
The Moffitt researchers observed a significant increase in the levels of EGCG in the blood plasma of men on Polyphenon E, and the capsules at this dose were tolerated in this group of men.
The ASCO poster session will take place Monday, June 1, 1:15-4:45 p.m. in S Hall A. The study was published in the April 14 issue of the journal Cancer Prevention Research. Funding support was received from the National Institutes of Health/National Cancer Institute (R01 CA12060-01A1).
Other items from the annual meeting of the AUA | THE "NEW" PROSTATE CANCER INFOLINK
Gene Mapping of Advanced PCa gives New Hope
Genetic study could provide hope for men with advanced forms of the disease with findings that could lead to personalised treatments
culture. Scientists have produced a map of genetic
mutations linked to the disease.
Photograph: Paul Hakimata / Alamy/Alamy
Prostate cancer ‘Rosetta stone’ paves way to targeted drugs | Society | The Guardian
Scientists unveil prostate cancer's 'Rosetta Stone' - Medical News Today
Almost 90 per cent of men with advanced prostate cancer carry genetic mutations in their tumours that could be targeted by either existing or new cancer drugs, a landmark new study reveals.
Scientists in the UK and the US have created a comprehensive map of the genetic mutations within lethal prostate cancers that have spread around the body, in a paper being hailed as the disease's 'Rosetta Stone'.
Researchers say that doctors could now start testing for these 'clinically actionable' mutations and give patients with advanced prostate cancer existing drugs or drug combinations targeted at these specific genomic aberrations in their cancers.
The study was led in the UK by scientists at The Institute of Cancer Research, London, in collaboration with researchers from eight academic clinical trials centres around the world.
Uniquely, doctors at The Royal Marsden NHS Foundation Trust and at hospitals in the US were able to collect large numbers of samples of metastatic cancers - cancers that had spread from the original tumour to other parts of the body.
Normally these samples are extremely hard to access, and this is the first study in the world to carry out in-depth analysis of metastatic prostate cancers that are resistant to standard treatments.
The research is published in the major scientific journal Cell, and is funded by Stand up to Cancer and the Prostate Cancer Foundation.
High Weight linked with Poor PCa Outcomes
Increasing body mass index linked with worse localized prostate cancer outcomesFox Chase Cancer Center : Increasing Body Mass Index Linked with Worse Localized Prostate Cancer Outcomes
The median age of patients was 68 years and the median radiation dose was 78 gray. Patient BMI distribution was as follows:
- About 20 percent of the included patients had a BMI of less than 25 kg/m2,
- 48 percent had a BMI of 25 to 29.9 kg/m2,
- 23 percent had a BMI of 30 to 34.9 kg/m2,
- 6 percent had a BMI of 35 to 39.9 kg/m2, and
- 4 percent had a BMI of 40 kg/m2 or greater.
- Increasing BMI was found to be associated with a small but increased rate of prostate cancer relapse (3 percent) in men treated with external beam radiation therapy.
- In addition, increasing BMI was also linked with
- small but significant increases in distant metastases (7 percent),
- prostate cancer-specific mortality (15 percent), and
- overall mortality (5 percent).