Summary of April Meeting of IPCSG of San Diego
By Bill Lewis
DVD’s of our meetings are available in our library for $10ea. Refer to the index available in the library. They can also be purchased through our website: http://ipcsg.org Click on the ‘Purchase DVDs” button.
New concepts in the screening and conservative management (Active Surveillance) approaches for Prostate Cancer
Chief
Scientific Officer, Genesis Healthcare Partners
Voluntary
Clinical Professor, Department of Urology, UCSD
Background: Prostate cancer is the second leading cause
of cancer-related death. Although the
percentage of men diagnosed with prostate cancer in their lifetime has almost
doubled from 9% to 17% since 1990, when the PSA test was widely adopted for
screening, the percentage of men that actually die of prostate cancer is now
only about 3%. Due to PSA screening,
there are 40% fewer deaths from prostate cancer than before. Many of the men now diagnosed with prostate
cancer each year have "low risk disease," and never would die of it,
but still undergo active treatment that is unnecessary. In May 2012, the U.S. Preventative ServicesTask Force recommended against PSA screening, due to frequent overdiagnosis and
overtreatment. The PIVOT (prostate
intervention vs observation trial) suggested that "observation" was
as effective as surgery, in July 2012.
But that applied only to low risk PCa, and there was a significant
benefit for patients with high risk prostate cancer.
The
Task Force recommendations were widely adopted, resulting in a 50% decline in
PSA screening, and in turn resulting in fewer diagnoses of low risk PCa (as is
desirable, since such cancers normally don't need to be treated), but an
increase in finding high risk PCa. This suggests
that men are not being biopsied as early in their disease progression, due to
the reduced frequency of PSA screening, and Dr. Gaylis and colleagues published
a letter in the New England Journal of Medicine in February 2016 pointing this
out, and warning that doctors may now be missing the window of curability for
many men -- as was common in the pre-PSA era.
Just
this month, the Task Force updated their recommendations to indicate that the
decision whether or not to test for PSA must be "individualized" for
men aged 55 - 69 years (including both men at average risk, as well as those
who are at “increased risk,” such as African American men, and those with a
family history of prostate cancer). They
still oppose testing of men aged 70 years and older, indicating their opinion
that the benefits do not outweigh the harms.
A major factor in the Task Force revision of their guidelines is the
fact that whereas in 2012, very few men went on active surveillance, now almost
40% do after diagnosis (thus avoiding the harms of overtreatment). The American Urologic Association immediately
issued a statement in favor of the revised guideline. Supporting evidence for the value of
screening comes from the European Randomized Study of Screening for Prostate
Cancer, which issued the prediction that 3 men out of 1000 will avoid
metastatic prostate cancer because of screening.
Dr.
Gaylis feels that men at "increased risk" (see above) should start
PSA testing much earlier than age 55; as early as age 40. And he also believes that the age 70 cutoff
is artificially rigid, and that each man's situation should be considered
individually. The popular term is
"shared decision making." He
has been doing this throughout his career, and notes that our support group
helps men to be involved in the decision making.
Active
surveillance can be considered to relate to "time of treatment," or
"delayed treatment." The
careful follow-up recommended by Genesis Healthcare includes an annual digital
rectal exam (because very aggressive cancer may not produce PSA; Dr. Gaylis
found a half-inch tumor in the prostate of a man with a PSA of only 0.7),
regular PSA testing (useful for the vast majority), genomic testing (such as
Oncotype Dx, Prolaris, and Decipher), multiparametric MRI, and biopsies.
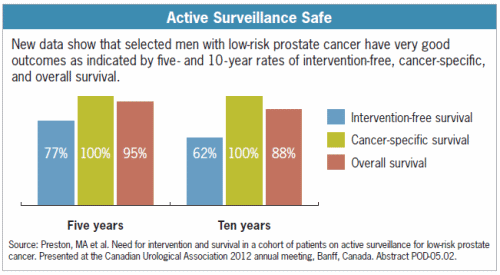
About
33% of men on active surveillance will eventually require active treatment to
prevent harm from the disease. Only 3%
of men with favorable-risk PCa who go through active surveillance (with active
treatment if and when needed) will die of the cancer within 10 years. The risk of dying from some other cause was
19 times higher than the risk of dying from PCa! (Source:
J. Urol. Suppl., 2009,181:606 abstract 1682)
At
Genesis Healthcare, for five years now, a "best practice" standard of
care involving treatment both of "very low risk" men, as well as more
liberal criteria for reasonable exceptions to the most strict criteria has been
used and shared with other doctors and groups.
Their data was published, with their guidelines, in the “Gold Journal,”
Urology, last year. Now about 70% of
their "low risk" patients go on active surveillance, and more than
85% of "very low risk" patients likewise. The definition of “very low risk” is as
follows: Stage T1c disease (identified
only by needle biopsy; not palpable by DRE nor visible by imaging); Gleason
Score = 6 or less; PSA less than 10 ng/ml; three or fewer biopsy cores
positive, and less than 50% cancer in any core; PSA “density” less than 0.15
ng/ml/gram (density = PSA score divided by the estimated weight of the whole
prostate). The more liberal criteria
amount to the patient’s request to go on active surveillance, with the risk
estimated as low and approved by the physician.
Genetic
testing discussion: Note that Genetics
examines the function of a single gene, whereas "Genomics" examines
groups of genes to identify their combined influence on an organism. The Oncotype DX prostate cancer assay was
discussed as an example of genomic testing.
It is suitable for newly-diagnosed men with very low, low, or
low-intermediate risk PCa. It helps to
improve assignment of the degree of risk, particularly to identify patients who
may need immediate treatment.
One
part of the Oncotype DX test evaluates the genes that affect the
"health" of the interaction of the prostate cells with the
surrounding stroma. Stroma is the
scaffold or supporting structure around the cells. Proliferation genes are used to measure
of how rapidly the cells turn over, or multiply. (Note that the Prolaris test only
looks at proliferation.) Androgen
signaling genes provide a measure of the cell's responsiveness to
testosterone. And cellular
organization is the fourth group of genes evaluated. All four groups of genes, 12 genes in all,
are compared with 5 reference genes that serve to account for varying RNA
quality and quantity in the test sample.
The assay provides an overall "Genomic Prostate Score" on a
scale of 100, with higher scores representing more aggressive cancer.
A
study at UCSF showed, that whereas 10% of a group of 288 men were thought to be
very low risk using the strict guidelines like Genesis Healthcare uses, after
considering the Oncotype DX score, it was found that 26% of the men could be treated
as very low risk. As might be expected,
given the uncertainties of biopsy results, some individual men found their GP
Score raised their predicted risk, while others found the opposite. Dr. Gaylis emphasized that these tests
provide "additional information" that can be a valuable part of the
overall assessment of risk and treatment options for each man.
Dynamic
contrast enhanced (DCE) MRI detects the greater blood flow (more blood vessels)
in prostate tumors as compared with healthy tissue. Along with two other parameters measured
using MRI, a "PI-RADS" (Prostate Imaging Reporting and Data System)
score is generated, on a scale of 1 to 5, with 5 being "highly suspicious
of malignancy."
Fusion
biopsies (combining prior MRI data with real-time ultrasound to guide the
needles during the biopsy procedure) are now gaining acceptance, because they
reportedly detect cancer two to three times as often as standard (i.e.,
"systematic" but blind, often referred to as random) biopsies, and
are especially effective in finding cancer when the MRI images show a high level
of suspicion. This new approach provides
progress toward targeted, "pinpoint" biopsies.
A
study published last year showed that only 5-11% of men on active surveillance
after being diagnosed at age 66 or older during the years 2001-2009, received
follow-up testing that met the strict guidelines of the prominent PCa research
groups at Johns Hopkins and at Sunnybrook (Toronto, Canada), respectively. Hopefully, our group members are followed
more closely!
At
Genesis Healthcare, in collaboration with UCSD, three specific measures of the
quality of active surveillance activities now are: adoption (currently, 70% of qualified
candidates go on active surveillance), adherence (follow-up PSA testing; DRE
annually; and "confirmatory biopsy" within 18 months), and patient
satisfaction at their first consultation.
Questions/discussion: Comments on drugs used for patients on active
surveillance, such as Metformin, a statin, and Proscar? There is provocative info in the literature
that says that statins help prevent growth of prostate cancer. Some doctors are using these drugs. Xtandi is being used in a trial at Genesis
Healthcare, with patients on active surveillance. Also Casodex is being used by some. There is no data available yet that proves
the benefits of any of these scientifically.
Are
there oncologists at Genesis Healthcare?
They have radiation oncologists, but there are “political problems” with
adding medical oncologists, so they don't have any.
A
comment on economics: In the USA, we have an 18% inflation rate for medical
expenses, and those expenses currently total 18% of GDP. That’s about twice the rate in other 1st
world countries. The Task Force did a little good, trying to recommend against
unnecessary expenses, but their recommendations also had some bad effects. The AUA (American Urologic Association) only
recommends based on "scientific data," so they offer no
recommendations for many issues.
Value
of estradiol (an estrogen)? Estrogen
slows prostate cancer, but can cause strokes.
Differences
of other genomic tests compared to the Decipher test? Prolaris predicts 10-year survival, and is
based only on “proliferation promoting” gene activity. The GenomeDX “Decipher” test is useful to
predict if radiation would benefit a man who has adverse pathology (disease
outside of the prostate and/or PSA rising after prior treatment). All are RNA based test. The microarray technology of the Decipher
test looks at thousands of genes (others only look at hundreds), but shallowly
(low, med, high); it is especially good at predicting recurrence after
prostatectomy.
Dr.
Gaylis is not in favor of MRI before biopsy, but wants 12-core first, then
later a fusion biopsy and (for low risk patients) genomic testing to confirm
the original biopsy findings.
A
major factor in the Task Force revision of their guidelines is the fact that
whereas in 2012, very few men went on active surveillance, now almost 40% do
after diagnosis (thus avoiding the harms of overtreatment).
TURP
effect on future treatment, for men on active surveillance? Depends on how aggressive the surgery
was. A new technique is to vaporize the
tissue, which he thinks may reduce introduction of cells into the bloodstream. He has tried green and red light laser, but
didn't like them. Now there is a
technology using steam. Another new
technology is the PlasmaButton from ACMI, which coagulates the tissue, and
seems worthwhile.

Final%20.png)
No comments:
Post a Comment